Cyanoacrylate adhesives

Cyanoacrylate drop-in adhesives, known as CA adhesives for short, are one of the very strong adhesives used to bond metals (such as zinc, brass, aluminum, and iron), plastics, rubber, ceramics, tiles, stone, wood, MDF, and more.
-Features
These adhesives have a low viscosity,Cyanoacrylate adhesives are fast and very strong cyanoacrylate adhesives. Cyanoacrylate adhesives have a short shelf life, being usable for up to one month after opening and up to one year after production at room temperature. Cyanoacrylate adhesives are also slightly toxic. These adhesives are compatible with many materials.
-Applications
Given the speed of adhesion and transparency of these adhesives, they can be used in homes and household applications to factories and industrial applications. Cyanoacrylate adhesives are a versatile adhesive that is also used to bond ceramics, stone, MDF, pottery, etc.

These adhesives are sold under trade names such as INT 502, drop glue, etc.These adhesives are known in global markets as Super Glue, which is famous for its fast adhesion properties.
A very small amount of moisture on the surface can cause the adhesive to curing very quickly (in less than 2 seconds). The most common cyanoacrylate monomer is ethyl 2-cyanoacrylate.
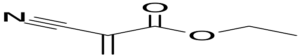
-Polymerization reaction of cyanoacrylates:
In general, C=C groups undergo chain growth polymerization rapidly in the presence of hydroxide ions, forming long and strong chains.
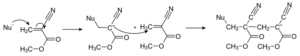
-How Cyanoacrylate Adhesives Work
The drying reaction of this class of adhesives is anionic polymerization, which is caused by small amounts of moisture on surfaces.
-Solvent for cyanoacrylate adhesives
When using these adhesives, it should be noted that the adhesive strength of cyanoacrylate is very high and if cyanoacrylate adhesives are attached to the skin, there is a possibility of skin damage. In these situations, solvents and vegetable oil can be used to separate the adhesive from the skin. Solvents that can be used include: acetone, nitromethane, dimethyl sulfoxide and dichloromethane.
References:
Ullman’s Encyclopedia of Industrial Chemistry
Degarmo Materials and Processes in Manufacturing, 13th Edition